November/December  2021
2021
IPPS: What's New and What's Ahead
By Jane Hulting, RHIA, CCS
For The Record
Vol. 33 No. 6 P. 18
To say there’s a lot going on with the inpatient prospective payment system would be quite the understatement.
Throughout the HIM profession, the effects of the COVID-19 public health emergency are far reaching. Coding departments need to look no further than the fiscal year (FY) 2022 inpatient prospective payment system (IPPS). While the final rule comes with the expected complement of new diagnostic and procedural codes and Medicare severity diagnosis-related group (MS-DRG) adjustments, many changes required recalibration to eliminate the exceptions of FY 2020.
IPPS Payment Rate Changes
The IPPS operating and capital market baskets were rebased and revised to reflect a 2018 base year, resulting in a 2.7% increase. The table below summarizes the percentage increases for IPPS with the applicable adjustments for quality data submission and meaningful EHR user status.
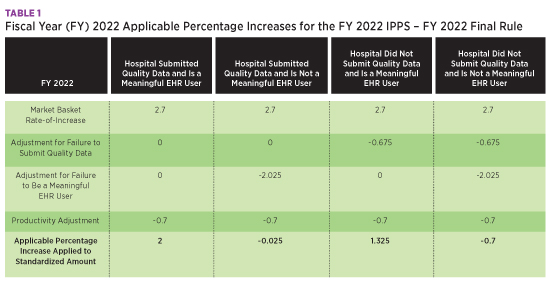
The Centers for Medicare & Medicaid Services (CMS) projects Medicare Disproportionate Share Hospital and Medicare uncompensated care payments to decrease in FY 2022 by approximately $1.4 billion from FY 2021. Overall, CMS estimates hospital payments will increase by $2.3 billion.
Quality Measure Changes
CMS established a policy for FY 2022 to suppress several performance data and quality measures affected by the pandemic, including the following:
• third and fourth quarters of calendar year (CY) 2020 Healthcare-Associated Infection (HAI) and PSI 90 data from Hospital Readmissions Reduction Program (HRRP) calculations (also to be suppressed for FY 2023);
• Hospital Consumer Assessment of Healthcare Providers and Systems survey;
• Medicare spending per beneficiary;
• five HAI measures;
• National Healthcare Safety Network (NHSN) Catheter-Associated Urinary Tract Infection Outcome Measure;
• NHSN Central Line-Associated Bloodstream Infection Outcome Measure;
• American College of Surgeons/Centers for Disease Control and Prevention (CDC) Harmonized Procedure Specific Surgical Site Infection Outcome Measure;
• NHSN Facility-Wide Inpatient Hospital-Onset Methicillin-Resistant Staphylococcus aureus Bacteremia Outcomes Measure; and
• NHSN Facility-Wide Inpatient Hospital-Onset Clostridium difficile Infection Outcome Measure.
The following are suppressed for FY 2023:
• 30-Day, All-Cause, Risk-Standardized Readmission Rate Following Pneumonia Hospitalization;
• Pneumonia 30-Day Mortality Rate measure;
• exclude COVID-19–diagnosed patients from denominators for calculation of all HRRP measures; and
• remove the Patient Safety and Adverse Events Composite measure.
New quality measures include the following:
• Maternal Morbidity Structural measures. Encourage standardization of protocols addressing obstetric emergencies and complications arising during pregnancy and childbirth;
• COVID-19 vaccination coverage among health care personnel measure. Allows CDC tracking of the number of health care personnel eligible to work in the hospital or facility for at least one day during the reporting period and who received a complete vaccination course against COVID-19;
• Hybrid Hospital-Wide All-Cause Risk Standardized Mortality measure. Voluntary reporting period is July 1, 2022, through June 30, 2023, followed by mandatory reporting beginning July 1, 2023, through June 30, 2024, affecting the FY 2026 payment determination and potentially affecting payment determinations for subsequent years;
• Two medication-related adverse event electronic clinical quality measures (eCQMs) beginning with the CY 2023 reporting period/FY 2025 payment determination;
• Hospital Harm-Severe Hypoglycemia eCQM; and
• Hospital Harm-Severe Hyperglycemia eCQM.
ICD-10-CM Code Changes
FY 2022 includes several new codes to report conditions that either have been unreportable or inaccurately reported since the inception of ICD-10-CM. Consider internal audits during the transition period until coders have demonstrated appropriate use of the new codes. A review of applicable query templates may also be in order.
F32.A Depression, unspecified
A code for unspecified depression had not been available in ICD-10-CM until FY 2022. Previously, unspecified depression was coded as F32.9, Major depressive disorder, single episode, unspecified. F32.A now allows the correct data representation of patients with unspecified depression as well as patients with major depression. Since coders are accustomed to assigning F32.9 for both unspecified depression and major depression, reporting both codes appropriately is essential to avoid denied claims.
I5A Non-ischemic myocardial injury (nontraumatic)
The detection of myocardial injury has increased in frequency due to widespread use of high-sensitivity troponin assays. Before this code was created, documentation of "myocardial injury" without specification of myocardial infarction or trauma was not reportable. Of note, this new code has been added to the complication or comorbidity (CC) list for FY 2022.
K22.81 Esophageal polyp and K22.82 Esophagogastric junction polyp
Prior to FY 2022, no specific codes existed for either esophageal polyp or esophagogastric junction polyp. K22.81 and K22.82, respectively, now capture these conditions.
P00.82 Newborn affected by (positive) maternal group B Streptococcus (GBS) colonization
If their mother tests positive for the bacteria during pregnancy, newborns are at increased risk for GBS disease during delivery. Previous coding advice instructed to use code P00.2 Newborn affected by other maternal circulatory and respiratory diseases for a baby born to a GBS-positive mother. However, this code is indicative of an active maternal infection and not appropriate when the mother is a carrier only. Code P00.82 now allows accurate reporting.
ICD-10-PCS Code Changes
Every fiscal year, ICD-10-PCS code changes report ever-changing surgical technology. FY 2022 is no exception.
Open Approach for Transfusions
For FY 2022, all codes in the Administration section, table 302 with Open approach, for body systems 3 Peripheral Vein, 4 Central Vein, and 8 Vein (87 codes), were deleted for clinical validity.
Cardiovascular Procedures
A new row was added to table 02F, Fragmentation of Heart and Great Vessels, for coronary intravascular lithotripsy (eg, Shockwave C2), a procedure that uses short pulses of sound waves to crack intimal and medial calcium within the vessel wall. Select the body part based on the number of vessels treated, and also code the stent insertion following the lithotripsy.
Table 02R, Replacement of Heart and Great Vessels, has new device characters to report the two types of artificial heart systems. Device character L Biologic with Synthetic Substitute, Autoregulated Electrohydraulic is selected for the CARMAT artificial heart system, while device value M Synthetic Substitute, Pneumatic is the choice for the Syncardia system.
The Harmony Transcatheter Pulmonary Valve procedure is performed at the native site of the valve. A new qualifier, M Native Site, was added to table 02R, Replacement of Heart and Great Vessels, to report this procedure. This device was approved for a maximum New Technology Add-On Payment of $26,975 for FY 2022.
New Technology table X2C with root operation Extirpation is now available for computer-aided mechanical aspiration thrombectomy, such as the Penumbra Indigo Aspiration System. This single-use, temporary system is indicated for the removal of fresh, soft emboli and thrombi from the peripheral arterial and venous systems as well as for treatment of pulmonary embolism.
Table X2K was created for a percutaneous arteriovenous fistula procedure known as the Ellipsys Vascular Access System, or EndoAVF procedure, using thermal resistance energy and ultrasound guidance.
A row was added to New Technology table X2R for the Thoraflex Hybrid device, a single-procedure, dual-purpose medical device that replaces the ascending aorta and aortic arch while also stabilizing and repairing the descending thoracic aorta.
New Technology table X2J has been added to report the use of the Caption Guidance system, transthoracic echocardiography with computer-aided image acquisition. This new procedure gives real-time feedback to the user on expected diagnostic quality of the resulting clip for possible additional images. For FY 2022, use of this system was approved for a $1,868.10 maximum New Technology Add-On Payment.
The Neovasc Reducer System is a device implanted in the coronary sinus using minimally invasive techniques to treat disabling refractory angina. A new row in New Technology table X2V has been added to report this procedure.
Musculoskeletal Procedures
Vertebral body tethering can help arrest and correct spinal curvature deformities in pediatric patients with scoliosis while allowing normal growth. The Tether Vertebral Body Tethering System uses a strong flexible cord to pull on the outside of a scoliosis curve to straighten the spine. To report this procedure, tables 0PS and 0QS for Reposition of Upper and Lower Bones have a new Device value 3, Spinal Stabilization Device, Vertebral Body Tether.
Codes were added for posterior dynamic distraction, a treatment for adolescent idiopathic scoliosis, in New Technology table XNS. The ApiFix Minimally Invasive Deformity Correction System enables permanent curve correction while retaining spine flexibility with a less invasive approach.
Scoliosis and other spinal deformities, such as kyphosis or lordosis, may be treated with another new technology, the aprevo devices, which tailor the deformity to the individual patient. Patient-specific intervertebral body fusion is reported with codes in New Technology table XRG. Aprevo devices are approved for a maximum $40,950 New Technology Add-On Payment for FY 2022.
New Technology code XW0V0P7 is now available to report the use of antibiotic-eluting bone void filler, such as Cerament G, as treatment for osteomyelitis. This product is specifically engineered as a single product to avoid a second-stage surgery.
Central Nervous System Procedures
A new row was added to table 4B0, Measurement and Monitoring of Physiological Devices, to identify the new device FlowSense. This device noninvasively and wirelessly assesses the flow of cerebrospinal fluid through an existing shunt for patients with hydrocephalus.
Gastrointestinal Procedures
New Technology table XD2 is used to report tissue oxygen saturation imaging of the gastrointestinal tract to visualize hemoglobin oxygen saturation levels to identify tissue that is not appropriately oxygenated and possibly ischemic.
New Technology code XDPH8K7 is available to report colonic irrigation for colonoscopy for patients who are unable to adequately prep due to comorbidities or motility issues. Pure-Vu System, Motus system, cleansing system, colonoscope oversleeve, or cleansing sleeve are documentation terms to watch for.
Table XFJ was added for reporting single-use duodenoscope using EXALT Model D and the aScope Duodeno during endoscopic retrograde pancreatography. These instruments eliminate the need for reprocessing and reduce the risk of patient-to-patient transmitted disease due to ineffective processing. A maximum New Technology Add-On Payment of $1,715.58 was finalized for each of these devices for FY 2022.
Use New Technology code XWHD7Q7 to report Phagenyx System insertion for pharyngeal electrical stimulation, a procedure for neurogenic dysphagia.
Skin Procedures
Bioengineered allogeneic cellularized scaffold, eg, StrataGraft, mimics the normal function of native skin and promotes healing in severe burn patients. Because it is not patient-specific, it may be applied universally to patients. To report this procedure, a new row was added to table XHR with device value F Bioengineered Allogeneic Construct. A maximum New Technology Add-On Payment of $44,200 was finalized for this new graft.
The table below summarizes the New Technology Add-On Payments approved for FY 2022.
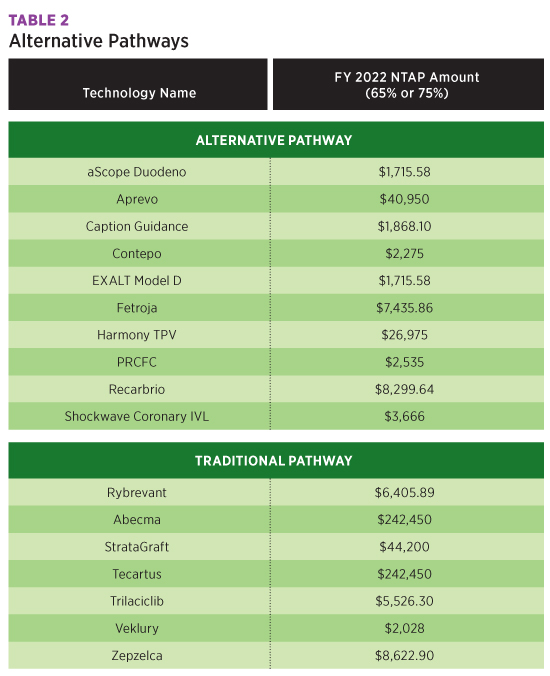
All New Technology Add-On Payments approved for FY 2021 will continue in FY 2022, and new COVID-19 Treatments Add-on Payment will be extended through the end of the fiscal year in which the public health emergency ends, including those approved for New Technology Add-On Payments for FY 2022.
Technology Group 1 Codes
New Technology codes in section X undergo review three years after their creation. All Technology Group 1 codes in Section X, added in 2018, were deleted, with one exception. Previous codes for orbital atherectomy, such as the DIAMONDBACK 360 Coronary Orbital Atherectomy System in table X2C, were deleted and are now reported in table 02C, Extirpation of Heart and Great Vessels, with new qualifier value 7 Orbital Atherectomy Technique.
MS-DRG Realignment
O.R. Procedure Unrelated to Principal Diagnosis
CMS annually reviews procedure codes, by volume, that result in MS-DRGs 981–983 (Extensive O.R. Procedure Unrelated to Principal Diagnosis) or MS-DRGs 987–989 (Non-extensive O.R. Procedure Unrelated to Principal Diagnosis) to determine whether their designation should change to a surgical MS-DRG and whether extensive O.R. or nonextensive O.R. is still appropriate.
The following procedure codes were reclassified from Extensive O.R. Procedure to Non-Extensive O.R. Procedure for the Unrelated to Principal Diagnosis MS-DRGs and have lower relative weights:
• Excision of subcutaneous tissue from the chest, back, and abdomen (0JB60ZZ, 0JB70ZZ, 0JB80ZZ);
• Laser interstitial thermal therapy (31 codes);
• Repair of esophagus via percutaneous, via natural or artificial opening, or via natural or artificial opening endoscopic (0DQ53ZZ, 0DQ57ZZ, 0DQ58ZZ); and
• Drainage of urethra, open approach (0T9D0ZZ).
For FY 2022, the following MS-DRG reclassification from this section resulted in a substantial relative weight increase: Control of bleeding in the cranial cavity (0W310ZZ, 0W313ZZ, 0W314ZZ) when reported with a central nervous system diagnosis moved from MS-DRGs 981–983 to MDC 01 in MS-DRGs 23 (RW 5.6719), 24 (RW 3.9390), 25 (RW 4.4974), 26 (RW 3.0620), and 27 (RW 2.5143).
O.R. to Non-O.R. Procedures
The following procedures were reclassified as non-O.R. for FY 2022, regrouping these procedures into lower-weighted MS-DRGs:
• Table 0J9 Drainage of subcutaneous tissue and fascia, open approach - 22 codes;
• Drainage of vestibular gland, open approach - 0U9L0ZX and 0U9LXZX;
• Insertion of feeding device into stomach, open approach - 0DH60UZ; and
• Table 0TC Extirpation of matter from the kidneys, kidney pelvis, and ureters, via natural or artificial opening endoscopic - six codes.
Non-O.R. to O.R. Procedures
The following procedures were reclassified as O.R. procedures, resulting in a relative weight increase:
• Table 0BB Excision of pleura, open approach, diagnostic - two codes;
• Table 0WC Extirpation of matter from upper/lower jaw, open/percutaneous endoscopic approach - four codes;
• Table 0SS Reposition right/left sacroiliac/hip joint with internal fixation device, percutaneous approach - four codes;
• Tables 0RH and 0RP Insertion/removal of spacer into right/left shoulder joint, open/percutaneous endoscopic approach - eight codes;
• Revision of intraluminal device in great vessel/upper artery/lower artery/upper vein/lower vein, percutaneous approach - five codes; these codes were also reassigned MS-DRGs in MDC (Major Diagnostic Category) 05; and
• XW0Q316 Introduction of eladocagene exuparvovec into cranial cavity and brain, percutaneous approach, new technology group 6.
A comprehensive, systematic review of ICD-10-PCS procedure codes is in progress. Watch for more reclassifications for O.R. and Non-O.R. procedures in future rulemaking.
Other MS-DRG Adjustments
External heart assist devices, such as the Impella Ventricular Support System, provide hemodynamic stabilization intraoperatively and for patients experiencing cardiogenic shock. Cases with device used intraoperatively (codes 02HA0RJ, 02HA3RJ, 02HA4RJ) were moved from MS-DRG 215 Other Heart Assist System Implant (RW 10.5584) and to MS-DRGs 216 (RW 10.0393), 217 (RW 6.4835), 218 (RW 6.1093), 219 (RW 8.0576), 220 (RW 5.4053), and 221 (RW 4.5799).
Reordered Surgical Hierarchy for MDC 05
Cases with multiple procedures are grouped to the highest surgical class in the hierarchy to which one of the procedures is assigned. In response to requests to examine MS-DRGs involving coronary artery bypass graft procedures with open ablation, four MS-DRGs in MDC 05 were reordered; MS-DRGs 231–236 Coronary Bypass are now sequenced above MS-DRGs 222–227 Cardiac Defibrillator with Implant.
MS-DRG Corrected Logic
Viral cardiomyopathy (B33.24) was moved from MDC 18, MS-DRGs 865 (RW 1.4778) and 866 (RW 0.8390) Viral Illness with and without MCC, to MDC 05, MS-DRGs 314 (RW 2.0847), 315 (RW 0.9734), and 316 (RW 0.7234) Other circulatory system diagnoses with/without MCC/CC, to be consistent with the MS-DRG assignments for other viral carditis codes (B33.20–B33.23).
Fourteen procedure code combinations for the right knee were incorrectly grouped to different MS-DRGs than the identical combination of procedures for the left knee and have been realigned.
Sweeping Changes Ahead
More than 3,400 unspecified codes are currently included in the CC or MCC list when more detailed codes on the list exist (eg, laterality). CMS has postponed removing these codes from the CC/MCC lists for FY 2022. To allow facilities time to prepare for this change, an edit has been added into the Medicare Code Editor for FY 2022 when an "unspecified" diagnosis code from the CC/MCC list is coded. To aid coders in capturing specificity without having to query the provider for this documentation detail, the Official Guidelines were updated for FY 2022 to include laterality in the list of items that can be reported from documentation by other clinical staff.
CC and MCC Review Is in Progress
The last comprehensive analysis of CCs and MCCs was completed in 2008. When an analysis was conducted for the FY 2020 proposed rule, extensive changes were recommended. Evaluation of the numerous comments received is ongoing. Watch for changes to the CC/MCC lists in future rulemaking.
MS-DRG Classification Split
Also underway is a review of all base MS-DRGs for possible subgroup creation. Five criteria are used to evaluate MS-DRGs for addition of a subgroup; all must be met to make a new two-way or three-way split. The proposed FY 2022 rule stated that, using this methodology, 96 MS-DRGs would be deleted and 58 would be created. Considering the impact of such extensive changes in light of the ongoing public health emergency, these MS-DRG reclassifications were delayed until FY 2023.
Review of the Final Rule, in concert with the tables of code changes and notes from past Coordination and Maintenance Committee meetings and others, allows facilities to be as prepared as possible, now and in the future.
— Jane Hulting, RHIA, CCS, is the senior director of education quality assurance for nThrive. She has more than 30 years of health care experience as a manager, consultant, coder, and auditor. She has acted as liaison and educator within clinical documentation improvement and collaborated with physician-adoption teams to improve EHR documentation.



