January-February  2021
2021
Telehealth Caretakers
By Patricia S. Coffey, RHIA, CPHIMS, CPHI, BS
For The Record
Vol. 33 No. 1 P. 22
Learn how the HIM department at a hospital dedicated to clinical research played a major role in its rapid deployment of telehealth.
The National Institutes of Health Clinical Center (NIH CC) is a 200-bed hospital that provides clinical care and clinical research support for 17 of the 27 Institutes and Centers of the NIH Intramural Research Program (NIH IRP). All research participants admitted to the NIH CC are enrolled in a research protocol and considered true partners in the research process.
The NIH CC supports 1,600 active clinical research protocols; approximately 50% of these studies are Phase I or Phase II clinical trials. This large portfolio of “proof of principle, first in human” research studies requires a keen focus on patient safety, timely and effective clinical care, and meticulous clinical and research documentation. Many team members, including 1,200 licensed independent practitioners, 600 clinical and research nurses, and social work, rehabilitation and spiritual care staff, support clinical research activities. In 2019, there were 25,124 distinct medical record numbers indicating either an inpatient visit or a scheduled appointment. The organization does not provide billing, emergency department, labor and delivery, or newborn nursery services.
At the onset of the COVID-19 pandemic, the NIH CC took measures to restrict clinical and research activity to only those that were urgent or lifesaving. These included limiting nonurgent new admissions to protocols, canceling elective surgeries, instituting telework broadly, and introducing additional environmental and administrative infection control measures to the work environment to reduce the risk of COVID-19 transmission.
While hospital operations were reduced and travel restrictions further impacted patient flow, the NIH CC, like other health care facilities, was tasked with developing strategies to safely provide clinical care and critical research support to research participants.
Telehealth has emerged as a central strategy in health care’s efforts to maintain continuity of clinical operations during the pandemic, resulting in the industry as a whole adopting the technology at a faster rate than what was occurring prepandemic.
Telehealth is defined as the use of electronic information and telecommunication technologies to support long-distance clinical health care, patient and professional health-related education, and public health preparedness. Telehealth technologies offer several advantages for the NIH CC while removing the need for physical contact that would lead to the reduction or limiting of potential infectious exposures. The NIH CC quickly recognized that a telehealth platform could easily support both its clinical research and patient care–related needs by enabling clinical research teams to meet virtually with patients to determine suitability for new studies as well as follow up with research participants already enrolled in studies.
Research Parameters
The NIH CC is a unique environment of clinical care provided in the context of research. As such, there are special considerations to protect the research participants and the research itself. As an organization focused on clinical research, the NIH IRP has a high level of interaction with its research participants and focuses on face-to-face communication while the participant is at the NIH CC.
Due to social distancing during the COVID-19 pandemic, there was a need to provide a telehealth platform and process at great speed. Multiple research and clinical teams across the 17 NIH institutes requested telehealth solutions to continue to screen, recruit, consult, and evaluate research participants. After defining the telehealth process and goals, technical tools were rapidly developed and leveraged in order to implement an initial telehealth program.
In an effort to ensure that the NIH CC could seamlessly continue to provide high-quality and safe clinical care while maintaining the integrity of the clinical research studies, the organization set out to design and implement a strategy that supported both clinical research team members and research participants. The following critical functions were identified to ensure continuity of clinical and research operations:
• the functionality to support virtual meetings between clinical research team members, inpatients, and outpatients—wherever they may be—to evaluate, provide care, and maintain research study compliance;
• the provision of visual and verbal exchanges between clinical research team members and research participants;
• the assurance that the platform is secure, easy and quick to implement, and accessible from any mobile device or computer; and
• the deployment of a system support model within the organization to assist both clinical research team members and research participants.
This article discusses the interdisciplinary process deployed to support long-distance clinical and research care in the NIH CC in a secure, confidential, and consistent manner that is user friendly to clinical research team members and research participants, and results in complete and timely documentation in the EHR.
Process
A crucial first step in the implementation process was to identify a technology that provided the required security and privacy controls in a platform that was readily available to staff within the NIH IRP program. A member of the informatics team investigated the functionality of MS Teams and how best to deploy it for telehealth. Following the identification of a suitable platform, the informatics team created a formal business plan for the development of an organizational telehealth program.
A central focus of the business plan was the identification of various scenarios highlighting how telehealth could effectively support both the provision of clinical care and the integrity of clinical research. The team tested MS Teams to ensure that it would be acceptable to both the clinical research team members and the research participants while supporting the NIH CC’s dual mission.
The second step was to define how to support telehealth across the organization. Barriers to telehealth implementation include the need for technical training of providers and patients as well as the ability to ensure available equipment for both parties. The opportunity to provide feedback and receive technical support has been shown to aid in the acceptance and patient satisfaction of telehealth.
Guided by the literature and internal discussions with key clinical research team members and the NIH CC informatics and HIM stakeholders, the project team determined there was an organizational need for a concierge.
The NIH CC’s HIM department took a lead role in defining the roles and function of the concierge service, which included the following:
• coordinating the scheduling of appointments;
• providing educational and other reference materials for clinical research team members and research participants;
• contacting and educating research participants about the telehealth process;
• assisting and/or meeting with research participants regarding technology requirements, including downloading the MS Teams application, and ensuring accessibility to the application using the appointment link and adequate wireless network or data support;
• assisting clinical research team members with telehealth documentation-related issues; and
• providing tablets to research participants who are in the NIH CC for use during telehealth appointments.
Other Innovative Functions
The NIH CC developed a notification mechanism to initiate a request for concierge services. The scheduling component of the EHR was leveraged to identify that the requested appointment is a telehealth visit. Clinical research team members schedule telehealth appointments by entering a “Virtual Visit Electronic Appointment Request,” preferably no less than 24 hours in advance of the appointment. Once entered, the concierge service contacts the patient to coordinate the visit. The appointment is then added to the clinical research team members’ Outlook calendar.
The process for scheduling a telehealth appointment is as follows:
• A clinical research team member contacts patients to ensure they are interested in and willing to participate in a telehealth appointment.
• A clinical research team member submits an electronic appointment request for the telehealth visit in the NIH CC EHR, known as CRIS (Clinical Research Information System), which automatically creates an appointment.
• HIM telehealth concierge service receives the request and schedules the telehealth visit; the appointment is added to the clinical research team member’s Outlook calendar.
• The telehealth concierge service includes details/instructions for the clinical research team member in the calendar appointment describing how to initiate the telehealth visit.
• Up to two days prior to the appointment, the telehealth concierge service contacts the research participant to perform a technical readiness assessment.
• If the technical readiness check is not successful, the telehealth concierge service contacts the clinical research team member.
• Once scheduled, research participants are able to view their scheduled appointments in the organization’s research participants portal.
At the time of the scheduled appointment, the clinical research team member launches the telehealth visit using their NIH account credentials. The research participant joins as a guest.
The process for joining telehealth appointments is the following:
• A clinical research team member launches the telehealth visit link.
• The telehealth concierge service instructs the research participant to join the telehealth visit 10 minutes prior to the appointment time to ensure a successful connection.
• Clinical research team members are instructed to utilize their normal processes for visits with research participants. For example, if the team normally calls patients two days after their visit to check on them, that process should continue and be documented in a telephone contact note in CRIS.
• Clinical research team members are also instructed to verify patient identity as they normally would.
Clinical research team members also have the ability to schedule interpreter services for telehealth visits. Like many services that are typically done in person, interpreters also are working remotely during the pandemic. When an interpreter is required for a virtual visit, the telehealth concierge service includes the NIH CC’s social work department’s interpreter services group e-mail address in the meeting invitation. A social work staff member then arranges with the interpreter services contractor to schedule an interpreter to attend the visit.
Once the interpreter is secured, the telehealth concierge service forwards the telehealth visit calendar invitation to enable them to join the encounter to provide interpretation services in the patient’s preferred language. In addition, telehealth visits are scheduled with interpreters for research participants who are onsite at the NIH CC to reduce the amount of staff needing to meet in person with research participants.
At the conclusion of the telehealth visit, a clinical research team member documents the visit in CRIS—just as they would for an onsite visit. This information is available to the patient in a secure patient portal. Clinical research team members can utilize existing documentation templates in CRIS to document the telehealth visit but must indicate in the note that it was a telehealth visit. Also, documentation must be completed by the end of the day of the visit.
HIM staff reviews all telehealth visits and updates the status of the appointments as “completed” or “not kept” using documentation from the clinical research team members. If no documentation is available, HIM contacts the clinical research team member to determine the appointment status.
Visits missing documentation are noted as a deficiency, tracked, and reported by HIM to the medical executive committee.
Policy
The organizational expectation is that all clinical care, clinical research, and consultation services provided via telehealth will meet the same safety and quality standards required during in-person clinical encounters and will comply with all appropriate policy statements, regulatory requirements, and accreditation standards.
In an effort to guide and ensure consistent implementation of the new telehealth program, an organizational policy statement was developed and approved by the medical executive committee.
The policy addresses the following issues:
• the determination of which clinical research team members may use telehealth;
• the scope of clinical care and clinical research activities that may be conducted via the telehealth platform (eg, some research protocols may not allow the use of telehealth for first protocol visits). The NIH CC supports the following activities: clinical care and clinical research activities, recruitment, screening, ambulatory care visits for evaluation, treatment, protocol requirements, and clinical consultation or patient support incidental to research;
• the type of telehealth approaches allowed—the NIH CC currently supports only synchronous activities;
• credentialing/privileging requirements. At the NIH CC, all clinical research team members approved by their supervisors can perform telehealth-related activities;
• the portability of licensure;
• documentation of patient consent for telehealth activity;
• explicit guidance about prohibited activities. For example, at the NIH CC, prescribing of controlled substances is not permitted in the context of a telehealth visit;
• a frequently asked questions resource and a point of contact for all potential users;
• a review of the process for conducting telehealth activities. The NIH IRP requires the use of the concierge service;
• guidance regarding documentation requirements;
• importance of the security and privacy controls necessary for telehealth activities;
• guidance regarding the optimal physical environment to ensure an effective and positive telehealth engagement; and
• any issues related to third-party reimbursement and/or billing. This issue is not germane to NIH CC because research participants are not billed for services provided.
Assessment and Lessons Learned
During the two-week early adoption phase, 20 different care teams utilized the program for approximately 60 telehealth visits. Of those visits, 10 were deemed incomplete primarily due to the patients not joining the meeting at the time of the visit because of technical issues on their end. HIM’s telehealth concierge service implemented processes to mitigate these issues, such as reminding research participants to use the same device that was tested during the technical readiness check and advising care teams to contact HIM for real-time assistance at the time of visit if they are experiencing technical issues.
As additional care teams utilized the telehealth program, other areas of improvement were identified. For example, if an electronic appointment request was entered for a same-day visit, HIM was not able to prepare adequately. To ensure timely notification of an impending (same day) telehealth appointment, an e-mail notification is now sent immediately to the concierge service from the EHR.
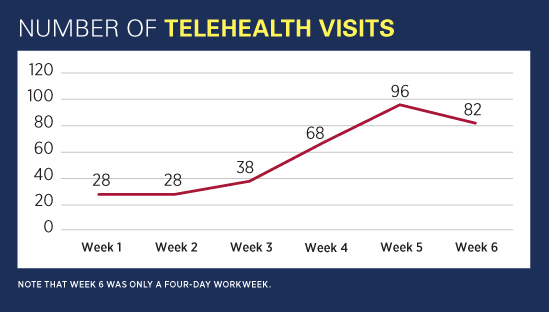
Upon implementation, the number of weekly appointments rose sharply (see Figure). The organization is continuing to identify other opportunities for improvement in order to focus on enhancing services for research participants who do not have access to e-mail or mobile devices.
The telehealth concierge service provides iPads to research participants who are inpatients at NIH CC and who do not have access to mobile devices. A generic e-mail address that is accessible on the device is provided to patients to use for the telehealth visit.
Next Steps
In the spirit of continuous improvement, the NIH CC is focusing on several areas for enhancement. Whereas the deployment of MS Teams has provided a reliable and effective platform, the NIH CC is investigating the use of platforms specifically engineered for telehealth interactions.
While MS Teams is a flexible tool that is serving the NIH IRP needs well, future work will require exploring its integration with the EHR as well as other platforms that provide telehealth functionality. The organization is hopeful that such a platform will be integrated within the EHR and the patient portal for ease of use by both research participants and clinical research team members.
Additional work is underway to improve the scheduling component in the EHR so that clinical services can have customized appointment requests rather than one generic version. This will allow clinical research team members to view their telehealth appointments on their own schedules, as well as improve capabilities within the EHR to stratify telehealth visits by clinical research team members and team.
Telehealth-specific documentation templates in the EHR are also under consideration. This would make documentation of these visit types easier for the clinical research teams while also ensuring the research and clinical needs of the services are met.
The Future
The NIH CC successfully established a synchronous telehealth program in a compressed timeframe that met the clinical care and research needs of both clinical research team members and research participants. The use of telehealth is expected to continue even after pandemic restrictions are lifted as the NIH CC IRP evaluates protocol requirements and explores opportunities to meet the requirements without expensive and unnecessary travel for the research participants it supports.
— Patricia S. Coffey, RHIA, CPHIMS, CPHI, BS, is chief of the HIM department at the National Institutes of Health Clinical Center. Coauthors include Marisa S. Owens, RHIA; Yvonne Almazon; Seth D. Carlson, MSIS, BCS; Amanda Grove, RHIA; Maria D. Joyce, MBA, CPA; Laura M. Lee, MS, RN; Cory Stephens, MSN, RN-BC, CPHIMS, SHIMSS; Susan M. Martin, RN, JD, CIPP-G, CPHIMS; Jon W. McKeeby, DSc, MBA, CPHIMS, CPHI; and Jennifer Wilder, RN, BSN, OCN, CHTC.
Funding statement:
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. [HHSN261200800001E 75N910D00024, Task Order No. (TBD) or HHSN261201500003I, Task Order No. (TBD)]. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Resources
1. 42 U.S.C. §254c-16(a)(4); The Health Care Safety Net Amendments of 2002 (P.L. 107-251).
2. Almathami H, Win KT, Vlahu-Gjorgievska E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients' homes: systematic literature review. J Med Internet Res. 2020;22(2):e16407.
3. Reeves J, Hollandsworth H, Torriani F, et al. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J Am Med Inform Assoc. 2020;27(6):853‐859.
4. Valentino LA, Skinner MW, Pipe S. The role of telemedicine in the delivery of healthcare in the COVID-19 pandemic. Haemophilia. 2020;26(5):e230-e231.
5. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962.



